In August 2011, the U.S. Department of Agriculture’s, Agricultural Marketing Service (USDA-AMS) released a draft of the “European Health Certification Program” to dairy industry trade associations (NMPF, the U.S. Dairy Export Council, the International Dairy Foods Association and the American Dairy Products Institute) for review and comment. After gathering member feedback, NMPF submitted comments and questions to AMS, expressing general support for the program and requesting additional clarification on specific points.
On November 21, 2011, USDA-AMS met with industry stakeholders, including NMPF, to discuss the logistical details of the project and to review the stakeholders’ remaining questions. As a result of that meeting, USDA-AMS finalized the requirements of the “European Health Certification Program.”
The effective date for beginning the transition to the new program requirements is January 1, 2012. After March 31, 2012, all shipments of dairy products requiring an E.U. health certificate must comply with the updated certification program and must be accompanied by an updated Certificate of Conformance.
The major differences between U.S. and E.U. milk requirements are:
1. The E.U. somatic cell count (SCC) and bacterial standard plate count (SPC) requirements apply at the farm level
2. E.U. maximum SCC in raw cow’s milk is 400,000 cells per mL.
Additional highlights of the program include:
• Milk suppliers, dairy processors and applicants for E.U. health certificates are responsible for maintaining records to trace their product back one step in the supply chain (toward the raw milk production) for all dairy products/ingredients intended for export to the E.U.
• Processors of dairy products/ingredients that require an E.U. health certificate will be responsible for maintaining Certificates of Conformance (COCs) demonstrating the dairy products/ingredients meet E.U. SCC and SPC requirements.
• Testing of the farm-level milk supply will be necessary to document compliance with the requirements for export of dairy products to the E.U. (both Grade A and Grade B milk for SCC, and Grade B milk for SPC).
Grade A plants that supply ingredients or raw milk are generally exempt from requirements to keep additional records on SPC to confirm compliance with E.U. regulations.
• Milk suppliers will be responsible for providing COCs to processors, as well as maintaining records of individual farms, to confirm raw milk meeting SCC and SPC requirements of the E.U. is received at facilities manufacturing dairy products for shipment to the EU.
• With respect to timing and implementation, all farms will be given three months to establish initial rolling three-month means – that is, SCC data collected in January, February and March will be used to determine the rolling three-month mean for April.
Non-Grade A farms will be given two months (January and February) to establish initial rolling two-month means for SPC. This data will serve as the initial basis for updated COCs under the new program requirements. According to the new program instructions, if a rolling mean exceeds E.U. requirements, the milk supplier must then notify AMS.
• The program instructions include a level of flexibility for farms that exceed E.U. SCC or SPC requirements, but work toward compliance.
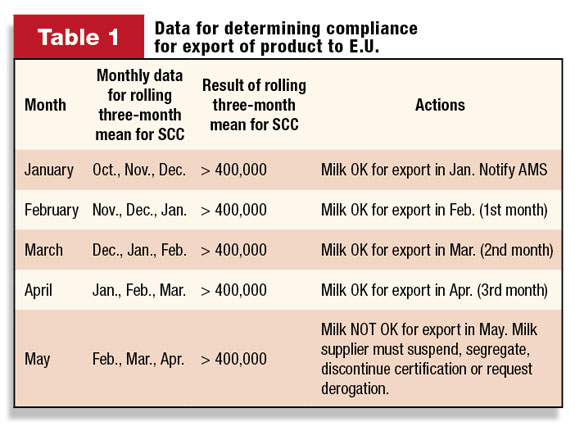
If a farm’s SCC mean or SPC mean exceeds the E.U. requirements for three consecutive months after the above notification to AMS Dairy Programs, as demonstrated in Table 1 , AMS would expect the milk supplier to take steps to request derogation or exclude the milk from E.U. certification when they receive the April numbers (in early May).
AMS will accept derogations as applying retroactively if the milk supplier makes the request in a reasonable time frame.
The milk supplier must do one of the following:
1. Suspend pickup of milk from the farm
2. Segregate the products made from that milk from the products that comply with E.U. requirements
3. Discontinue certifying that products made with this milk meet the requirements of Regulation (EC) No 853/2004
4. At the request of the farm, contact AMS Dairy Programs and ask for a derogation (deviation under special circumstances) to allow this milk to be accepted into the E.U. export program.
AMS will charge the milk supplier an administrative fee of two hours at the current Federal Register published rate for Dairy Program services for each derogation (seasonal or otherwise) application. This fee will cover the costs of administering and keeping the records for these derogations.
A derogation will be granted provided that during processing the milk or milk products are pasteurized or made into raw milk cheese that will be aged at least 60 days before being placed on the market. Corrective actions and out-of-compliance monitoring activities are expected to continue during the derogation period, which is valid for one year.
In the event of a farm’s continued non-compliance, the derogation must be reapplied for one year following the issuance of the derogation.
5. A milk supplier, at the request of the farm, may request from AMS Dairy Programs a “seasonal derogation.” A seasonal derogation will be granted to a farm that can demonstrate for a majority (at least nine months) of the year they are in compliance with the E.U. requirements for somatic cell count; only due to seasonal variations is the farm’s somatic cell count escalated for a period of time during the year.
The farm must be able to demonstrate through records that this variation is truly seasonal and not the result of poor hygiene or sanitary procedures. All seasonal derogations will be reviewed during the AMS Dairy Programs’ review of records to verify compliance with E.U. regulations. Seasonal derogations must be renewed every three years. PD
For more information see http://www.ams.usda.gov/AMSv1.0/
—USDA-AMS press release



