Throughout my more than 30 years of studying fat feeding and lipid metabolism in ruminant species, the major emphasis was consistently on sources and feeding rates, but little attention was directed at fat quality. I quickly learned in the laboratory of Dr. Don Palmquist at Ohio State University that strict protocols were needed to protect the fat in agricultural samples from destruction during drying, grinding and extraction.
Yet, these concerns about fat destruction rarely seemed to carry over into the field. High-fat ingredients used for livestock feed were processed, stored and mixed under extreme temperature and humidity conditions, usually without any question of implications to fat quality.
Fat quality is a consistent threat in everyday life. Fat is constantly susceptible to damage, whether its frying food in cooking oil at home, storing distillers grains in commodity bins under extremes of heat and humidity or the fat degradation in body tissues. In broad terms, overexposure of fat to heat and air for prolonged periods of time causes the fat to become oxidized. Fat oxidation is often accompanied by a darkening in color, a bad smell and off flavors.
Many decades of scientific studies have been devoted to describing the chemistry of oxidation and its influencing factors. The chemical reactions of lipid damage are indeed complex and beyond the scope of this article. Instead, it is hoped that this article touches the subject of lipid damage with a broad brush to bring attention to quality of feed ingredients fed to livestock and their utilization by body tissues.
The process of lipid damage begins with understanding the basic structure of feed fats. The building blocks of fats are called fatty acids that are categorized as either saturated or unsaturated. Saturated fatty acids are chains of carbons held together by only single bonds. Unsaturated fatty acids have one or more double bonds in their carbon chains (Figure 1).
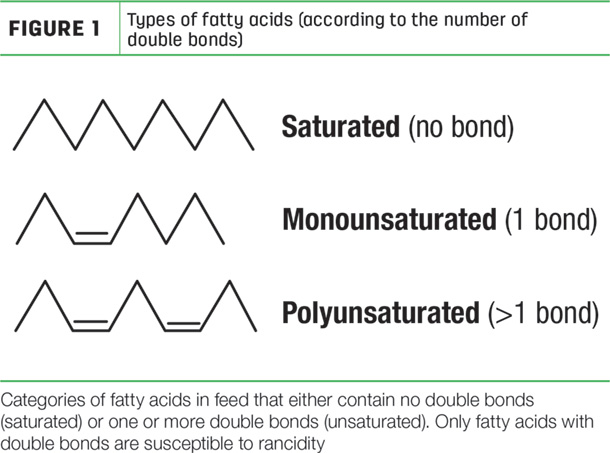
Plant-based fats found in most livestock feeds such as byproducts, cereal grains, soybeans and forages consist mainly of unsaturated fatty acids with multiple double bonds in their structures. These double bonds are susceptible to attack from oxygen in the air initiated by the presence of a catalyst. The reaction of oxygen with double bonds in unsaturated fatty acids form what is called free radicals. Free radicals then spontaneously react with double bonds on adjacent unsaturated fatty acids, forming more free radicals.
The process self-perpetuates as a chain reaction and is called peroxidation, resulting in a decline in unsaturation of the fat and an increase in peroxide concentration. Total peroxides are often measured as an indicator of the amount of rancidity. However, peroxides themselves eventually degrade into more than a dozen positional hydroperoxides. The extent this occurs in animal feed is not well documented, although many of the risk factors are often present, including unsaturated fatty acids, heat and light.
Prolonged storage of feed in the presence of these risk factors is likely to initiate peroxidation. Several factors found in feed and feed supplements enhance the risk of peroxidation, including fatty acids with three or more double bonds such as those found in forages, flaxseed and fish oil; a higher proportion of free fatty acids that were released from glycerol during harvesting and drying; and mineral supplements that contain copper and iron that catalyze peroxidation reactions.
Not to be ignored is the integrity of unsaturated fatty acids in body tissues of animals. In very simplistic terms, there is an ongoing battle within the cells of all animal species. Enemies produced from normal cell metabolism are trying to invade and damage critical cell functions but often are fought back by an internal defense system. In most circumstances, the defense system prevails over the enemies, preventing any cell damage. Thus, a balance of power is maintained. However, you might subject the animal to conditions that trigger the cells to overproduce the metabolic enemies, and the defense system becomes overwhelmed. This is an imbalance called “oxidative stress” and the end result is cell damage leading to disease or poor animal performance (Figure 2).
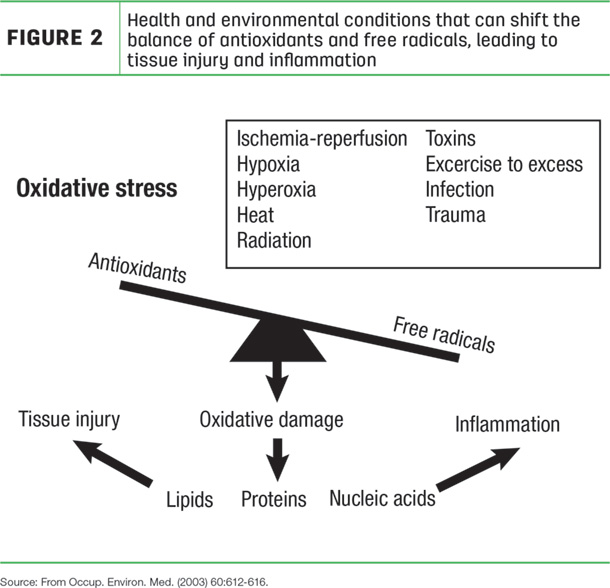
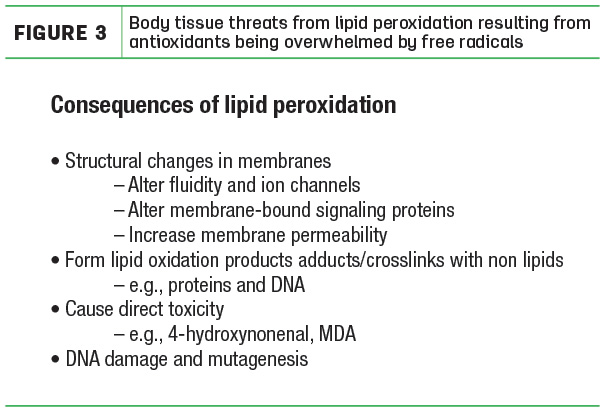
Why is the word “oxidative” used to describe the condition? It is because the cell enemies are made from oxygen. Every cell has a power plant called the mitochondria that uses oxygen to make the energy needed by the cell to sustain normal function and life. In fact, mitochondria use 85% to 90% of all the oxygen taken up by the cell. In the course of oxygen being metabolized to energy by the mitochondria, part of the oxygen has its structure rearranged. This altered oxygen is called the reactive oxygen species or free radicals. Small concentrations of free radicals are common and, in fact, useful to the cell, helping it to perform important everyday functions. However, uncontrolled increases in free radicals spread throughout the cell, destroying critical cell components (Figure 3).
The key to the prevention of peroxidation in feed and body lipids is an adequate supply of antioxidants. This topic will be addressed in Part 2.





