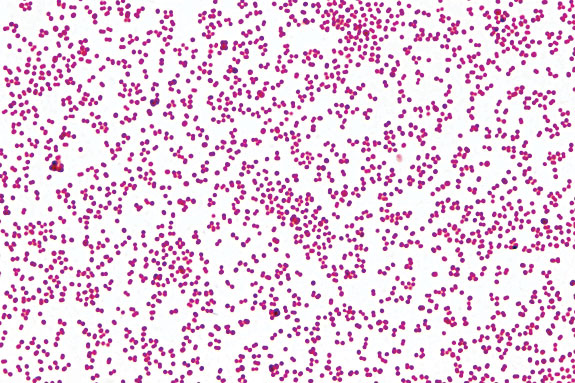
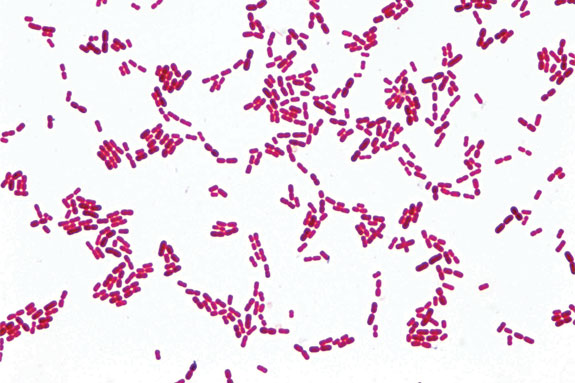
There has been a good deal of confusing information concerning the causative agent in bovine pinkeye over the past few years. Quality veterinary medical diagnostic laboratories around the country have reported the isolation of Branhamella ovis or Moraxella ovis from clinical cases of pinkeye patients. M. ovis is a spherical-shaped (coccoid) organism as opposed to the typical short “rod-shaped” Moraxella bovis.
For a number of years our diagnostic laboratory has been reporting isolations of spherical Moraxella species isolated from cases of bovine pinkeye as coccoid M. bovis. In genetic analyses of these organisms, we found that they were 97 percent identical to M. bovis and less similar to M. ovis; therefore, we believed these were a natural variant of M. bovis.
In 2007, Drs. Angelos and George (et. al.) at the School of Veterinary Medicine at the University of California at Davis reported that these spherical-shaped Moraxella organisms from cattle pinkeye cases represented a new species of Moraxella, Moraxella bovoculi.
Why is the naming of this species important? One reason, of course, is to clear up the confusion that has been so prevalent in our industry, so we can deal with the disease with some consistency and accuracy.
Another important reason is that there are currently no federally licensed, commercially available M. bovoculi vaccines. In a university herd of beef calves, Dr. John Angelos, Associate Professor of Medicine and Epidemiology at the University of California at Davis, College of Veterinary Medicine, found that M. bovoculi could be isolated more often from pinkeye-affected calves that had been vaccinated with M. bovis proteins compared to control (unvaccinated) calves.
Likewise, M. bovis was more often isolated from pinkeye-affected calves vaccinated with M. bovoculi proteins compared to control (unvaccinated) calves. These observations suggest that in herds where both M. bovis and M. bovoculi are present, it may be important to vaccinate for both organisms. We often culture cattle eyes with typical pinkeye lesions and isolate both M. bovis and M. bovoculi.
Of the bovine eye culture cases examined by our diagnostic laboratory from active pinkeye herds in 2010, we found only M. bovoculi in 42 percent, only M. bovis in 12 percent and both M. bovis and M. bovoculi in 26 percent. It should be noted that many of these herds had previously been vaccinated for M. bovis, which could explain why there were many more cases with M. bovoculi only.
Another important distinction is the susceptibility of these organisms to commonly used antibiotics. In our diagnostic laboratory we have found that M. bovis is still relatively sensitive to the antibiotics commonly used to treat pinkeye, but the same cannot be said for M. bovoculi, which appears to be significantly less sensitive to the commonly used antibiotics.
I often wonder how often we have clinical pinkeye that would yield a mixed M. bovis and M. bovoculi, but after a “failed” round of antibiotics we only isolate M. bovoculi due to its antibiotic resistance.
While I often recommend tetracyclines for treatment of pinkeye, we found that of the M. bovoculi on which we ran sensitivity testing, 37.84 percent were not sensitive to tetracyclines.
I am not aware of any large-scale efforts to look at this resistance phenomenon more closely. I would like to know if other diagnostic laboratories are seeing the same patterns of resistance.
For these reasons, I think it is important to have a reputable laboratory culture the eyes of animals with clinical pinkeye, especially if the herd has been properly vaccinated with commercial M.bovis vaccine.
I think it is also wise to request an antibiotic sensitivity test if your laboratory isolates M. bovoculi. It is expensive and frustrating to administer antibiotics to cattle and see no results due to an ineffective antibiotic.
Other than the differences discussed above, we assume that M. bovoculi and M. bovis affect cattle in very similar ways. So if we are going to minimize the effects of pinkeye disease in our cattle operations, we should review the following information and get to understand pinkeye disease.
Managing pinkeye in cattle herds
When referring to pinkeye, we are referring specifically to bovine eye disease caused by Moraxella bovis, Moraxella bovoculi or a mixed infection of the two. (In this article we will refer to the two species together as Moraxella spp.)
Since the bovine eye can only respond in a limited number of ways to disease or injury, there are many times when a case may be perceived as pinkeye yet not involve Moraxella spp.
Pinkeye is considered to be the single-most important ocular disease of cattle and occurs worldwide wherever cattle are raised. Economic loss due to pinkeye has been estimated to be up to $200 million per year in the United States alone.
This estimate is based on lack of gain in feedlots and on greatly diminished weaning weights (25 to 40 lbs per head) in calves. The estimate does not include time and money spent treating affected cattle eyes or lost milk production due to diminished lactation or milk discarded due to antibiotic residues.
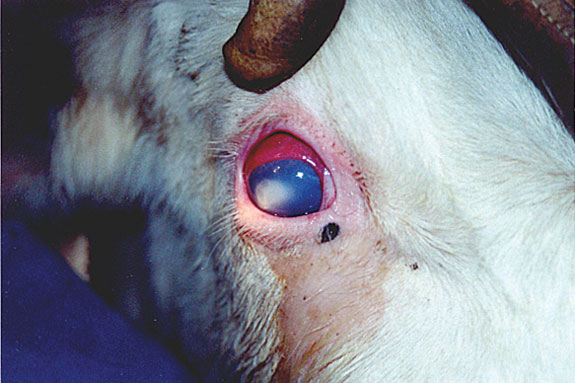
Clinical signs of pinkeye
The disease starts with eye tearing. Tearing is usually very evident from the inner corner of the eye and cattle will often squint in the affected eye. Usually within a day of the start of tearing, close examination of the eye will reveal pits (1/16 of an inch or larger) called lesions or ulcers of the surface (cornea) of the eye.
Over the next few days these may enlarge; the eye may turn blue and then white. There may be a blood-red border around these ulcerations. In severe cases, the surface of the eye may become cone-shaped and may rupture.
Animals affected this severely in both eyes can become blind. Blindness can often be prevented with early treatment and simultaneous vaccination (revaccination). Pinkeye normally starts with one or two animals and may quickly spread to the other animals by face flies or direct contact.
Usually within two weeks of the initial case, the herd outbreak will be at its most severe stage. Recovered cattle may have blue to white scars on their eyes and eye surfaces may be enlarged or misshapen.
Although pinkeye can and does occur at any time of year, it is primarily a seasonal disease with the greatest occurrence being associated with exposure to peak periods of ultraviolet light irradiation. Most outbreaks are associated with a number of predisposing factors which are very important to consider in managing and preventing outbreaks.
Predisposing factors
• Age of cattle: Calves experience significantly more pinkeye than do yearling cattle, which in turn have a higher rate of pinkeye than do older cattle. The assumption is that an acquired immunity is developed over time which to some degree is protective against Moraxella spp.
• Breed of cattle: Purebred cattle vary significantly as to their susceptibility to pinkeye. Herefords (especially those with unpigmented eyes) have the highest rate of pinkeye, while Angus and Zebus have the lowest rate.
It should be noted, however, that when Angus cattle do develop pinkeye, the lesions tend to be quite severe and recovery may often be incomplete.
Crossbred cattle also seem to have varying rates of pinkeye, e.g. Hereford cross with no Angus may have up to ten times the pinkeye rate when compared to Hereford cross with Aberdeen Angus.
Keep in mind that the eyes of cattle are covered with a thin layer of skin cells (cornea) and that the ability of Moraxella spp. to invade the eye may be dependent on some sort of damage to the cornea. We can look at other pinkeye predisposing factors that may be directly involved in damaging these cells.
• Ultraviolet irradiation: (bright sunlight) has been demonstrated to aid corneal colonization by Moraxella spp. and microscopic examination has shown the cell damage initiated by UV burning.
• Viral infection: (e.g. IBR virus) is capable of damaging the protective cells covering the eye, not only on the cornea but also on the eyelids.
• Physical trauma: Blowing dust and sand, weed seeds and stubble, face flies, tail switching, antibiotic powders, etc., can scratch the cornea and allow entry of Moraxella spp.
• Chemical trauma: Fresh nitrogen on the pasture can burn the protective cell layer.
A number of factors may interfere with the immune response in cattle. Once again, UV irradiation may play a role. It is well documented in the human population that excess UV exposure leads to a depressed immune status at least in the skin, if not overall.
Laboratory mice can have their immunity severely depressed by exposure to a sunlamp. Nutritional deficiencies in cattle can occur even under the care of the most well-meaning of managers.
Pasture and hay may look acceptable, yet on analysis be very low in vitamin A, zinc, selenium and copper, etc. Sometimes a little corn or mineral supplement can greatly improve the immune status of a herd.
It has been well documented that various viral agents can severely suppress the immunity of the host. It has been shown that BVD attacks the thymus, which is an important source of immune cell production in the animal.
Stress from shipping, processing, insects, etc., can be very immunosuppressive.
One other factor should be brought up for discussion as a possible predisposing factor and that is photosensitivity. Photosensitivity may be a result of ingestion of a toxic substance in the forage.
In the case of a mild photosensitivity that may be inapparent on the skin due to the hair coat, it has been suggested that the cells covering the eye may well suffer damage, thus allowing penetration of Moraxella spp. This may also help to explain why bordering pastures may have vastly differing pinkeye rates in the animals grazed on them. The photosensitivity possibility is strictly theoretical at this point, but I believe it justifies further exploration.
If we keep in mind that pinkeye caused by Moraxella spp. is a very contagious disease, it will help us to understand the method of spread and help us to practice more effective control measures. The bovine eye, nasal passages and sometimes the vagina are where the organism resides and presumably overwinters.
It is not unusual to find that cattle begin to break with pinkeye two to three weeks after processing in the spring. If we consider that the organism may be shed in high numbers in tears or nasal secretions and that the animals are crowded and extremely stressed during processing, we obviously have all the right circumstances for the spread of disease in general and pinkeye in particular.
It is not known how long Moraxella spp. can survive in the body secretions or on environmental surfaces, but it has been known to survive for up to three days on the feet of a face fly and, therefore, we should assume that all surfaces contaminated by infected animals (crowding chutes, clothing, instruments, trailers and barn walls) are potential sources of disease for at least several days.
Due to this contagious nature, handlers should be aware that contamination on their clothing, hands, ropes, etc., may be capable of transferring infectious agents during processing. Insects, especially face flies, should be considered to be mechanical vectors.
Aerosols (sneezing) have been incriminated in the spread of Moraxella spp. and I do not believe we can rule them out as possible modes of transmission, as cattle carry high numbers of organisms in the nasal passages.
Treatment of pinkeye
We feel that when an animal is run through the chute to be treated for pinkeye, we should do everything possible while the animal is being restrained in order to prevent having to get that patient up again. Common treatment procedures include:
1. Antibiotic therapy, either topically, eyelid injection or intramuscular. Topical application is easy to do but it is difficult to maintain effective levels of topical antibiotics in the face of heavy tearing. From a purely psychological view, many cattlemen seem to feel better when the cow has “purple stuff” on its face because they can see that they have at least done something for their animal.
IM injection of antibiotics such as long-acting tetracycline, has the added advantage of helping to reduce the numbers of M. bovis in other tissues in the body as well as the eye.
2. Protecting the eye from sunlight and other irritants may accelerate healing as well as make the patient more comfortable. Sutures, eye patches and isolation in a dark barn are all options to be considered.
3. Vitamin A can be injected and nutritional deficiencies (especially copper and selenium) should be addressed.
4. Vaccination with an M. bovis bacterin may boost the antibody response in previously vaccinated animals. Significant improvement in pinkeye cases have been observed as quickly as three to five days post- vaccination. I strongly recommend vaccination/revaccination when treating active pinkeye cases.
Prevention
Minimizing predisposing factors can be very helpful. Pay attention to fly control programs, clip pastures, perform forage analysis to help ensure adequate nutrition, provide shade and pay attention to the fact that we are dealing with a contagious disease.
Vaccination for M. bovis with some of the newer vaccines has proven to be very advantageous.
Other organisms have been associated with bovine eye disease and include Chlamydia, IBR virus, Mycoplasma bovoculi, Moraxella bovoculi, Listeria and Neisseria sp.
Pinkeye is an economically important disease in cattle worldwide. Having a more thorough understanding of pinkeye and educating ourselves on treatment and control measures can play an important role in positively impacting the profitability of raising cattle.
References omitted due to space but are available upon request to editor@progressivedairy.com.
PHOTOMICROGRAPHS and PHOTOS
Top: The tiny pink circular dots are the spherical-shaped Moraxella bovoculi.
Middle Top: The tiny rod-shaped organisms are typical Moraxella bovis. Images courtesy of Addison Labs.
Middle Bottom: Shows a typical eye that has pinkeye.
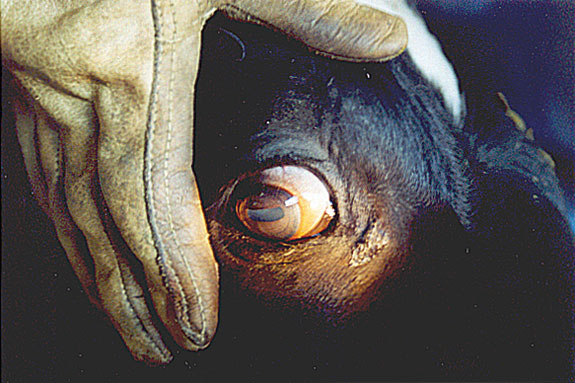
Bottom: Shows a normal eye. Photos courtesy of Addison Labs.






